| Quantum Mechanics Solution Manual |
|
© Leon van Dommelen |
|
4.3.3.1 Solution hydc-a
Question:
If there are infinitely many energy levels 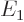 ,
, 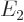 ,
, 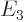 ,
, 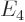 ,
, 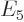 ,
, 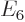 , ..., where did they all go in the energy spectrum?
, ..., where did they all go in the energy spectrum?
Answer:
The 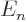 for large values of
for large values of  are all graphically indistinguishable from zero energy. The electron is almost free from the nucleus in those energy states.
are all graphically indistinguishable from zero energy. The electron is almost free from the nucleus in those energy states.
![]() ,
,![]() ,
,![]() ,
,![]() ,
,![]() ,
,![]() ,
,![]()
![]()