| Quantum Mechanics for Engineers |
|
© Leon van Dommelen |
|
14.4 Draft: Magic numbers
In nuclear physics, there are certain special values for the number of
protons or the number of neutrons that keep popping up. Those are the
values shown by horizontal and diagonal lines in the decay plot figure
14.2:
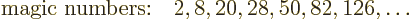 |
(14.2) |
These magic
numbers were historically found to be
associated with unusual stability properties. For example, the magic
number of 82 neutrons occurs in 7 stable nuclei, more stable nuclei
than for any other number of neutrons. The runners-up are 20 and 50
neutrons, also both magic numbers, that each occur in 5 stable nuclei.
Nuclei that have a magic number of protons also tend to have unusual
stability. For example, the element with the most stable isotopes is
tin, with 10 of them. Tin has 
 50 protons, a magic number.
To be sure, the runner up, Xenon with nine stable isotopes, has
50 protons, a magic number.
To be sure, the runner up, Xenon with nine stable isotopes, has 
 54, not a magic number, but the heaviest of these nine stable
isotopes has a magic number of neutrons.
54, not a magic number, but the heaviest of these nine stable
isotopes has a magic number of neutrons.
The last element to have any stable isotopes at all is lead, and its
number of protons 
 82 is magic. The lead isotope
82 is magic. The lead isotope
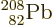 , with 82 protons and 126
neutrons, is doubly magic, and it shows. It holds the triple records
of being the heaviest nucleus that is stable, the heaviest element
that is stable, and the highest number of neutrons that is stable.
, with 82 protons and 126
neutrons, is doubly magic, and it shows. It holds the triple records
of being the heaviest nucleus that is stable, the heaviest element
that is stable, and the highest number of neutrons that is stable.
The doubly magic  nucleus, the alpha
particle, is stable enough to be emitted in alpha decays of other
nuclei.
nucleus, the alpha
particle, is stable enough to be emitted in alpha decays of other
nuclei.
Nuclei with magic numbers also have unusually great isotopic presence
on earth as well as cosmic abundance.
The reason for the magic numbers will eventually be explained through
a simple quantum model for nuclei called the “shell
model.” Their importance will further be apparent throughout
the figures in this chapter.
![]()
![]()
![]()
![]()
![]()
![]()
![]() ,
,![]()