|
|
|
|
|
Next: 6. Macroscopic Systems |
|
The electron states, or atomic orbitals
, of the
elements discussed in section 5.9 form the basis for the
valence bond
description of chemical bonds. This
section summarizes some of the basic ideas involved.
As pointed out in section 5.9, helium is chemically inert:
its outermost, and only, shell can hold two electrons, and it is full.
But hydrogen has only one electron, leaving a vacant position for
another 1s electron. As discussed earlier in chapter 5.2,
two hydrogen atoms are willing to share their electrons. This
gives each atom in some sense two electrons in its shell, filling it
up. The shared state has lower energy than the two separate atoms, so
the ![]()
Fluorine has one vacant spot for an electron in its outer shell just
like hydrogen; its outer shell can contain 8 electrons and fluorine
has only seven. One of its 2p states, assume it is the horizontal
axial state 2![]() ,
,![]()
This bond between p electrons looks quite different from the
![]()
sigma
bonds: if you
look at either bond from the side, it looks rotationally symmetric, just like an s
state. (Sigma is the Greek equivalent of the letter s; it is written
as ![]() .
.
Key Points
- Two fluorine or similar atoms can share their unpaired 2p electrons in much the same way that two hydrogen atoms can share their unpaired 2s electrons.
- Since such bonds look like s states when seen from the side, they are called sigma or
bonds.
The ![]()
![]() ,
,![]() ,
,![]()
![]()
However, the 2![]()
![]()
![]()
This covalent bond, and the corresponding one between the
2![]()
pi
or ![]()
So, the ![]()
Key Points
- Unpaired p states can match up sideways in what are called pi or
bonds.
Oxygen, located in between fluorine and nitrogen in the periodic
table, has two unpaired electrons. It can share these electrons with
another oxygen atom to form ![]() ,
,![]() O
O
In the water molecule, the lone 2![]()
Similarly, the lone 2![]()
Since the ![]()
![]()
The reason that the actual angle is a bit more may be understood from
the fact that the oxygen atom has a higher attraction for the shared
electrons, or electronegativity, than the hydrogen atoms. It will
pull the electrons partly away from the hydrogen atoms, giving itself
some negative charge, and the hydrogen atoms a corresponding positive
one. The positively charged hydrogen atoms repel each other,
increasing their angle a bit. If you go down one place in the
periodic table below oxygen, to the larger sulfur atom, ![]() S
S
Bonds like the one in water, where the negative electron charge shifts
towards the more electronegative atom, are called
polar
covalent bonds.
It has significant consequences for water, since the positively charged hydrogen atoms can electrostatically attract the negatively charged oxygen atoms on other molecules. This has the effect of creating bonds between different molecules called “hydrogen bonds.” While much weaker than typical covalent bonds, they are strong enough to affect the physical properties of water. For example, they are the reason that water is normally a liquid instead of a gas, quite a good idea if you are thirsty, and that ice floats on water instead of sinking to the bottom of the oceans. Hydrogen is particularly efficient at creating such bonds because it does not have any other electrons to shield its nucleus.
Key Points
- The geometry of the quantum states reflects in the geometry of the formed molecules.
- When the sharing of electrons is unequal, a bond is called polar.
- A special case is hydrogen, which is particularly effective in also creating bonds between different molecules, hydrogen bonds, when polarized.
- Hydrogen bonds give water unusual properties that are critical for life on earth.
While valence bond theory managed to explain a number of chemical bonds so far, two important additional ingredients need to be added. Otherwise it will not at all be able to explain organic chemistry, the chemistry of carbon critical to life.
Carbon has two unpaired 2p electrons just like oxygen does; the difference between the atoms is that oxygen has in addition two paired 2p electrons. With two unpaired electrons, it might seem that carbon should form two bonds like oxygen.
But that is not what happens; normally carbon forms four bonds instead
of two. In chemical bonds, one of carbon's paired 2s electrons moves
to the empty 2p state, leaving carbon with four unpaired electrons.
It is said that the 2s electron is promoted
to the 2p
state. This requires energy, but the energy gained by having four
bonds more than makes up for it.
Promotion explains why a molecule such as C![]()
However, promotion is still not enough to explain the molecule. If
the C![]()
![]()
![]() ,
,![]() ,
,![]()
![]()
The explanation is that, rather than using the 2![]() ,
,![]() ,
,![]() ,
,hybrid
states. (This is not unlike how the torus-shaped ![]()
![]()
![]()
![]()
In case of C![]() ,
,![]() ,
,![]() ,
,![]()
![]()
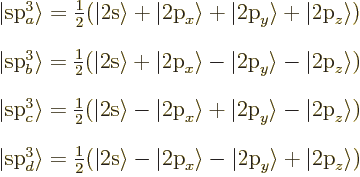
All four s![]()
The asymmetrical shape can increase the overlap between the wave
functions in the bond. The four s![]()
![]()
But carbon is a very versatile atom. In graphite, and carbon
nanotubes, carbon atoms arrange themselves in layers instead of
three-dimensional structures. Carbon achieves this trick by leaving
the 2p-state in the direction normal to the plane, call it
![]() ,
,![]()
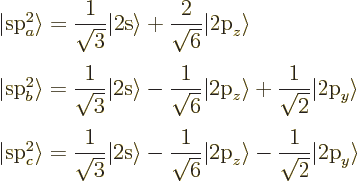
These planar hybrids are under 120 degree angles from each other,
giving graphite its hexagonal structure. The left-out p electrons
normal to the plane can form pi bonds with each other. A planar
molecule formed using s![]()
![]() H
H![]() )
)
Finally, carbon can combine the 2s state with a single 2p state to form
two sp hybrids under 180 degrees from each other:
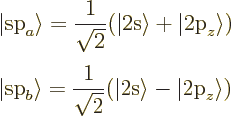
Key Points
- The chemistry of carbon is critical for life as we know it.
- It involves two additional ideas; one is promotion, where carbon kicks one of its 2s electrons into a 2p state. This gives carbon one 2s and three 2p electrons.
- The second idea is hybridization, where carbon combines these four states in creative new combinations called hybrids.
- In s
p hybridization, carbon creates four hybrids in a regular tetrahedron combination.
- In s
p hybridization, carbon creates three hybrids in a plane, spaced at 120 degree intervals. That leaves a conventional 2p state in the direction normal to the plane.
- In sp hybridization, carbon creates two hybrids along a line, pointing in opposite directions. That leaves two conventional 2p states normal to the line of the hybrids and to each other.
Ionic bonds are the extreme polar bonds; they occur if there is a big difference between the electronegativities of the atoms involved.
An example is kitchen salt, NaCl. The sodium atom has only one electron in its outer shell, a loosely bound 3s one. The chlorine has seven electrons in its outer shell and needs only one more to fill it. When the two react, the chlorine does not just share the lone electron of the sodium atom, it simply takes it away. It makes the chlorine a negatively charged ion. Similarly, it leaves the sodium as a positively charged ion.
The charged ions are bound together by electrostatic forces. Since these forces act in all directions, each ion does not just attract the opposite ion it exchanged the electron with, but all surrounding opposite ions. And since in salt each sodium ion is surrounded by six chlorine ions and vice versa, the number of bonds that exists is large.
Since so many bonds must be broken to take a ionic substance apart,
their properties are quite different from covalently bounded
substances. For example, salt is a solid with a high melting point,
while the covalently bounded C![]()
Chapter 10.2 will give a more quantitative discussion of ionic molecules and solids.
Key Points
- When a bond is so polar that practically speaking one atom takes the electron away from the other, the bond is called ionic.
- Ionic substances like salt tend to form strong solids, unlike typical purely covalently bound molecules like hydrogen that tend to form gases.
Valence bond theory does a terrific job of describing chemical bonds, producing a lot of essentially correct, and very nontrivial predictions, but it does have limitations.
One place it fails is for the ![]()
![]()
![]()
![]()
![]()
![]()
![]()
Valence bond theory also has problems with single-electron bonds such as the hydrogen molecular ion, or with benzene, in which the carbon atoms are held together with what is essentially 1.5 bonds, or rather, bonds shared as in a two state system. Excited states produce major difficulties. Various fixes and improved theories exist.
Key Points
- Valence bond theory is extremely useful. It is conceptually simple and explains much of the most important chemical bonds.
- However, it does have definite limitations: some types of bonds are not correctly or not at all described by it.
- Little in life is ideal, isn’t it?